Abstract
Introduction
Four direct oral anticoagulants (DOAC) have been approved for the treatment of venous thromboembolism VTE and prevention of stroke in patients with non-valvular atrial fibrillation (NVAF) in the USA: rivaroxaban, apixaban, edoxaban and dabigatran. Data regarding efficacy and safety of DOAC in the cancer population however are currently limited to very small cohorts in the large randomized trials for treatment of VTE in which many cancer patients did not meet eligibility requirements, or to retrospective studies and quality improvement initiatives. Despite major consensus guidelines recommending low molecular weight heparin (LMWH) as the preferred treatment for cancer-associated VTE, DOAC are commonly prescribed for cancer patients in both community and academic settings. Data on the safety and efficacy of DOAC in patients getting active cancer treatment are lacking. We aimed to characterize the use of DOAC in patients managed at an academic comprehensive cancer center, to examine the incidence of thrombotic and bleeding events among patients receiving active cancer therapy during the study period.
Methods
Following IRB approval, a retrospective chart review was performed in patients treated at an academic comprehensive cancer center. Patients >18 years old who had a prescription written for a DOAC between June 2015 and December 2016 were identified. Patients were included if they were receiving DOAC for NVAF, acute VTE, and secondary prevention and had active cancer. Patients were assessed for baseline demographics, indications for anticoagulation, DOAC management strategies, arterial and venous thrombotic events and bleeding complications during treatment with DOAC. Descriptive statistics were used to analyze study outcomes.
Results
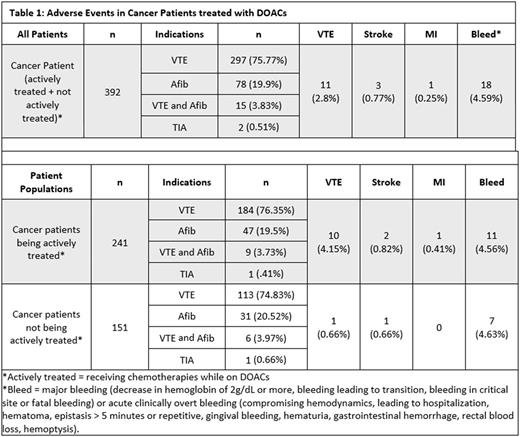
Of the 721 patients screened, 392 were patients with cancer of which 241 s were actively being treated with chemotherapy while on DOAC. The other 151 patients were not actively receiving treatment. VTE was the most common indication for DOAC use in 75% of cancer patients (n=297), followed by NVAF in20% (n=78). There were 15 patients with history of both NVAF and VTE, and 2 patients with TIA. Apixaban was used in 48% of patients; rivaroxaban in 47%; dabigatran in 4%, and edoxaban < 1%.
Of the 392 evaluated cancer patients, 15 (3.83%) experienced thrombotic events with 11/15 new or recurrent VTE, 3/15 stroke, and 1/15 myocardial infarction while on DOAC. The VTE rate was strikingly higher in those patients receiving active cancer treatment at 4.15% compared to 0.66% in those not getting treatment. The rate of major bleeding and clinically overt bleeding was not different between the 2 groups and was approximately 4.5%. (table 1)
Conclusion
We found in our retrospective analysis of DOAC use in cancer patients, that patients getting active cancer treatment had higher rates of primarily venous and arterial thrombotic events while treated with DOAC compared to those patients not getting active cancer treatment. The rate of VTE at 4.14% compares favorably with published rates for VTE recurrence for LMWH and VKA in randomized controlled trials and is roughly 7 times higher than the rate in our patients with cancer treated with DOAC but not getting active cancer treatment. Despite differences in rates of thrombotic events, the bleeding rates were the same, and also comparable to published rates, confirming the known increased risk of bleeding in cancer patients. While our results are promising, dedicated randomized controlled trials comparing DOAC to LMWH are required to truly determine the efficacy and safety of DOAC in cancer patients simultaneously receiving cancer treatment.
Connors: BMS: Consultancy, Other: Scientific Advisory Board; Pfizer/BMS -Alliance: Other: Independent review committe; Unum Therapeutics: Other: Data Safety Monitoring Board; boehringer Ingelheim: Other: Scientific Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal